Tissue outside the body: In vitro models in research
In vitro studies enable the investigation of biological processes outside of the organism. They allow science to obtain fundamental insights into the human body without the use of animal testing. Toxicity, cell communication, mechanical investigations – all of this can already be observed in cultivated tissue in vitro (Latin for “in a glass”).
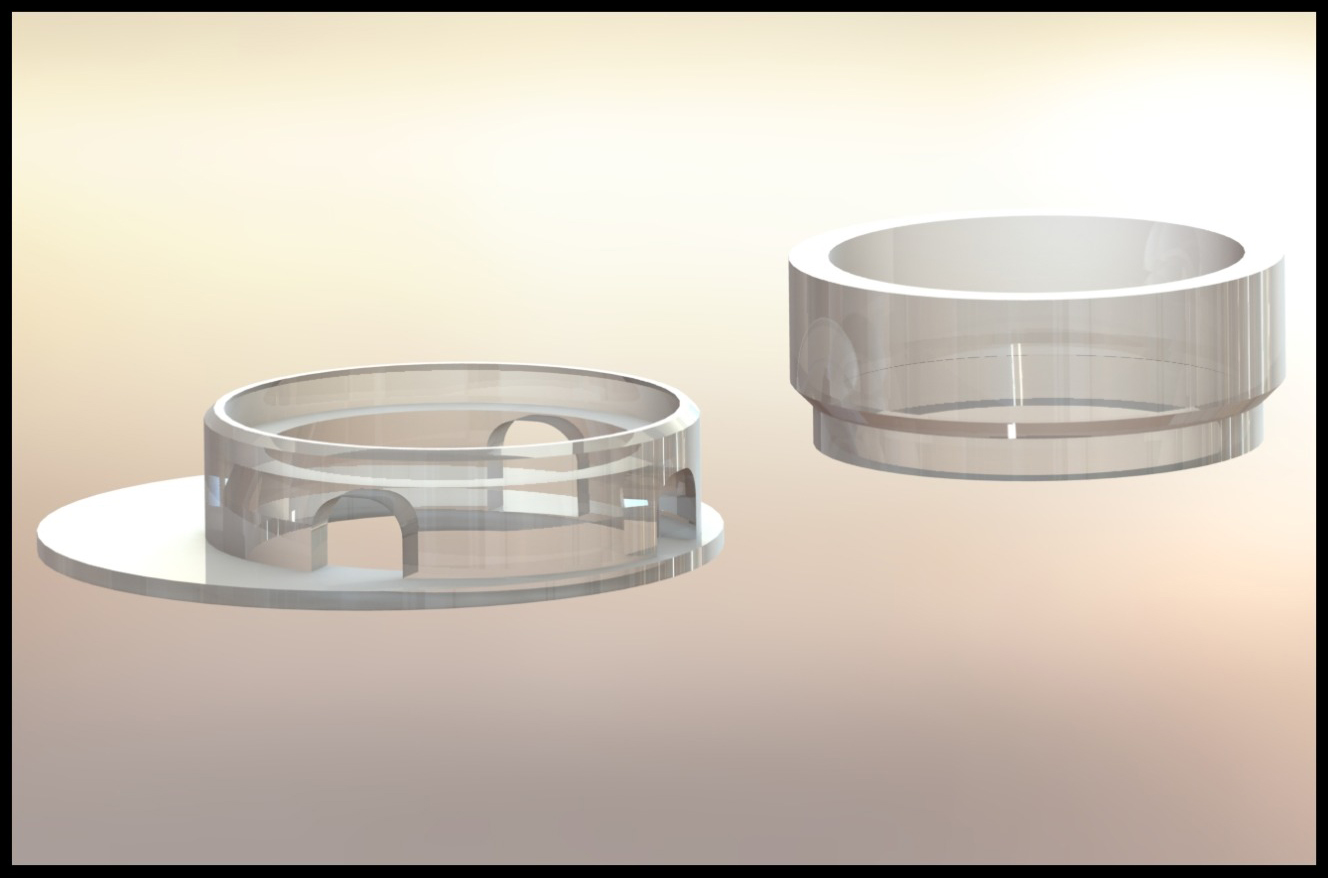
Indeed, imitating the body in vitro is not easy – and it can often be expensive. Skin models, for instance, are best studied in the shape of three-dimensional “skin equivalents”. The cultivated skin is stretched in a plate with small recesses – both the skin equivalents and the tensioning devices (called “inserts” because they are inserted into the recesses of the plate) can be purchased. This comes at a cost. To save valuable research budget in the future, while also improving the design of the inserts, Magdalena Bauer dedicated her master’s thesis to the development of 3D-printed inserts. She carried out her work in the group of Dr. Peter Dungel at the Ludwig Boltzmann Institute for Traumatology (LBI Trauma), the research center in cooperation with AUVA.
For the production of the inserts, she used PLA, short for Poly-Lactic Acid, a polymer made up of linked lactic acid molecules bonded together. PLA can be produced by fermenting molasses or by fermenting glucose, so unlike many other plastics, it can be obtained from renewable raw materials.
From the first prototype to the final functional version, six construction variants were used. Some requirements were specified from the beginning: the insert needed a ring for clamping the largest possible membrane as well as the ability to supply the membrane with nutrient medium. In skin equivalents, the nutrient medium is typically located beneath the skin – in imitation of the situation in the body, where the skin is also supplied from below. In the cell culture plate, the nutrient medium is pipetted to the side of the ring and finds its way under the skin through small openings.
Even the first prototype allowed the skin to be clamped between two rings that were perfectly matched in size. In following designs, these were moved to the edge to allow enough space for a serological pipette, which greatly facilitated the feeding process. Further improvements focused on the supply of nutrient solution and avoiding small air bubbles under the membrane. Finally, the upper ring was slightly widened to facilitate the removal of the skin for final analysis purposes.
The inserts were evaluated for cytotoxicity according to ISO 10993-5, and no toxic effects on cells were observed. To demonstrate the functionality of the new inserts, skin equivalents were grown from human skin cells on collagen/fibrin gel. Measurement of the skin barrier function was also possible in the 3D printed inserts. For this purpose, Magdalena Bauer added a dye to the solution below the skin equivalents. It was tested whether the dye could pass through the skin at different time intervals. If the skin equivalent was intact, this did not happen within the experimental time. However, if a dye and a substance that attacks the skin barrier were added to the solution below the skin equivalents, the dye could be detected on the other side of the skin after a short time – the barrier was disrupted. The substance added during this test was already known for its effect on the skin barrier – it was merely used to demonstrate that Magdalena’s 3D printed system is fully functional. In the future, new substances can also be tested for their effects on human skin.
In vitro models are also being optimised by other research groups at the LBI Trauma. Marian Fürsatz from the group for cartilage regeneration, which is located at the Vienna General Hospital in cooperation with the Medical University of Vienna, developed a novel system for the production of cartilage pellets. These pellets, which are only about one millimeter in diameter, consist of in vitro cultured cartilage and are widely used in studies of this tissue. Typically, they are cultured individually in small indentations on a plastic plate and are laborious to produce and maintain. In his dissertation under the supervision of Dr. Sylvia Nürnberger, Marian developed special cell culture plates in which the small cartilage pellets form spontaneously. A checkered pattern is engraved on the plates using a CO2 laser. The seeded cells – a co-culture of cartilage cells and mesenchymal stem cells from adipose tissue – initially grow two-dimensionally along the plastic surface. At a certain cell density, this cell lawn detaches from the surface and contracts. Marian takes advantage of this property. The engraved lines form a disruption in the cell lawn, along which it breaks apart – and a small micro-cartilage pellet forms spontaneously in each square.
The consumption of nutrient medium and the workload for medium exchange can be significantly reduced in subsequent cultivation, as many pellets can be cultured side by side in the same plate.
In numerous follow-up experiments, Marian was able to demonstrate that his pellets are comparable to those from conventional, labor-intensive production. They become just as round and show similar cell differentiation properties. They even showed better reproducibility. Whereas cell differentiation (as the maturation into fully functional cartilage cells including the production of new cartilage matrix) is often subject to variations from pellet to pellet in the conventional process, Marian’s pellets behave more homogeneously. The scientists suspect that this can be attributed to the fact that many pellets are in the same medium. Differences due to subpopulations can thus be better compensated, since the cells can still send signals to each other via the medium – even from pellet to pellet. This is not possible in the classical system. This means more reliable results thanks to the use of pellets as a model tissue in experiments. All this is able with less expensive cultivation and reduced labor.
The first study on this innovative system already appeared this year in the renowned journal Biofabrication.